MEASURING AND MODELLING THE DYNAMIC RESPONSE
OF REMOTE MOUNTAIN LAKE ECOSYSTEMS TO
ENVIRONMENTAL CHANGE
A programme of MOuntain LAke Research
MOLAR
PROTOCOL FOR
FISH SAMPLING:
TEST FISHING
FISH PHYSIOLOGY
FISH HISTOLOGY
HEAVY METALS
ORGANIC MICROPOLLUTANTS
Bjrrn Olav Rosseland
Sigurd Rognerud
Jean-Charles Massabuau
Rudolf Hofer
Joan O. Grimalt
Norwegian Institute for Water Research, Oslo
Universite de Bordeaux I et CNRS, Arcachon
University of Innsbruck
Department of Environmental Chemistry (CID-CSIC)
Protocol for fish population sampling
Bjrrn Olav Rosseland, NIVA, Norway
1. Test fishing
1.1 SNSF gillnet series
In AL:PE 1 and 2, the gillnet series that was design for the Norwegian monitoring programme (Rosseland et al. 1979) has been used (Wathne et al. 1995). Eight individual bottom gillnets of different mesh sizes (Table 1) or a set of three gillnets, each as a combination of these 8 mesh sizes (SFT 1983), has been used either alone or in combination.
The new series consist of three gillnets, 32 X 1.5 m, each net containing the 8 mesh sizes mixed and randomised in units of 4m. The same type of nets as in the single net SNSF series (thread size, colour etc.) are used, as well as the producer of the nets. Because of the different catch efficiency of the series, the old (8 single nets) was given a Catchability of 1, compared to 0.46 of the multimesh size series (3 nets).
Table 1. The SNSF gillnet series, containing 8 gillnets of given mesh size and threadthickness. (After: Rosseland et al. 1979). In the series of 8 single nets, each individual gillnet is 26 X 1.5 m, and have a dark red colour. In the SNSF multimesh-size series, 3 nets are a unit, 32 X 1.5 m each net containing a combination with 4 m panels of each mesh size. Each of the 3 nets in the unit have the individual mesh sizes in different order. The catchability of the 8 single nets are 1.0, compared to 0.46 of the 3 net series. The gillnets are produced by Lundgrens Fiskredskapsfabrik AB, Stockholm, Sweden.
Mesh size mm. 10 12.5 16.5 22 25 30 38 46
Thread thickness mm. .15 .15 .15 .15 .15 .15 .15 .17
1.2 The "Nordic" multimesh-size series
Multimesh-size gillnet series has also been used in other Nordic countries. In Sweden, a monitoring program was initiated in 1983 to study the long-term effects of liming on fish populations (Degerman et al. 1988). In this program, a multimesh-size gillnet series containing 14 randomly distributed panels of 4 x 1.5 m (total gillnet length 42 m) of mesh sizes from 6.25 to 75 mm was used (Hammar and Filipsson 1985).
In 1990, a co-operation between Norway, Sweden and Finland was established to develop sampling protocols for monitoring of fish populations in acidified lakes. As a part of this, a new "Nordic multimesh-size gillnet series" was developed, which since then has been tested against the "national standard" for each country. In Finland, the comparative studies started in 1993 (Kurkilathi and Rask, 1995), and in Norway in 1992 (Jensen and Hesthagen 1996, SFT 1993). The Nordic series contains
12 different mesh sizes, between 5 and 55 mm,, in panels of 2.5 m, height 1.5 m and total length of 30 m per net, Table 2.
Table 2 The Nordic multimesh-size gillnet series, contains 12 mesh size, each panel being 2.5 m long and 1.5 m in height, total gillnet length of 30 m. The mesh sizes and thread thickness are given. The gillnets are produced by Lundgrens Fiskredskapsfabrik AB, Stockholm, Sweden.
Mesh size mm. 5 6.25 8 10 12.5 16 19.5 24 29 35 43 55
Thread thickness mm. 0.1 0.1 0.1 0.12 0.12 0.15 0.15 0.15 0.15 0.18 0.20 0.25
In 1992 (SFT 1993, Figure 2), a comparative study on 8 brown trout and 5 Arctic charr (Salvelinus alpinus L.) populations in Norway, found the comparative CPUE values between the old SNSF series and the Nordic series to be:
Brown trout: NordicCPUE = 0.79 * SNSFCPUE + 3.18 (P<0.005)
Arctic charr: NordicCPUE = 1.62 * SNSFCPUE + 5.05 (P<0.01)
Figure 2 A comparison between the Catch per Unit Effort (CPUE) of the SNSF series and the Nordic series in lakes with populations of brown trout (Aure) and Arctic charr (Rrye). From SFT (1993).
In another comparative study using the SNSF and the Nordic series, the conclusion was stated:, "Over a limited range of mesh sizes and fish sizes (5-20 cm fish), the selectivity of the two types of net do not differ much" (Jensen and Hesthagen 1996).
Based on these results, there seems to be no problem by either the SNSF series or the Nordic series as standard gillnets in the MOLAR.
1.3 Gillnet-setting
The gillnets are set perpendicular to the shore, avoiding steep-slope shore to bottom areas. Gillnets in the "old" series are set at random as for mesh size, whereas the "new" series always will have all eight mesh sizes represented at a certain point.
Both in the AL:PE 1 and 2 projects, the period between August 15 to October 15 have been selected for testfishing, for reasons given in Wathne et al. (1995). This is the main (and only) period for providing data to describe the population structure of salmonids. However, in the MOLAR project, physiological and histological data from other periods of the ice free season are needed. Sub-sampling of fish will therefore take place in some localities from early summer to late autumn.
1.4 Analytical program
Each fish must be given a specific number which follow all subsamples to be analyzed. Data from individual fish must be sent to NIVA. Due to differences in age determination of fish from some lakes in the AL:PE 2 project, age determination of all fish used in the MOLAR project should be undertaken by one laboratory.
For each individual fish, the following parametres must be noted:
· lake
· date
· species
· length, in mm, measured from snout to lower part of tail.
· weight, in gram.
· sex and gonadal maturation, from stage I - VII
- I - II juveniles
- III - V recruit spawners
- VI spawning
- VII/.. postspawners
· flesh colour; white, pink or red.
· stomach fullness, classified from 0 - 4.
· stomach content (if possible), conserved in 70% alcohol and classified in main invertebrate groups (not fully analysed in AL:PE 2).
· scale samples from brown trout only for age determination, taken from the area between the sideline organ and dorsal and pectorial fin.
· otolith samples for age determination (all species), using the "burning technique" described by Christensen (1964). If age differ when determined by otolith and scales, otolith age is considered as the true age.
· growth, determined by:
- length at catch as a function of true age.
- back calculation of growth (brown trout only), using the methods of Dahl (1910) and Lee (1920).
Otholiths from single fish should be sent to NIVA for age determination. The results are processed in data bases at NIVA
Bjrrn Olav Rosseland
NIVA
Brekkevein 19
P.O. Box 173 kjelsls
N-0411 Oslo, Norway
Phone: 47 22185110
Fax: 47 22185200
bjoern.rosseland@niva.no
2. References
Christensen, J.M. 1964. Burning of otoliths, a technique for age determination of Soles and other fish. J. Cons. perm. int. Explor. Mer. 29, 73-81.
Dahl, K. 1910. Alder og vekst av hos laks og Rrret belyst ved studiet av deres skjCl. (Age and growth of Atlantic salmon and brown trout by use of scales). Landbruksdepartementet Centraltrykkeriet, Kristiania. (In Norwegian).
Degermann, E., Nyberg; P. and Appelberg, M. 1988. Estimating the number of species and relative abundance of fish in oligotrophic Swedish lakes using multi-mesh gillnets. Nordic J. Freshw. Res. 64: 91-100.
Hammar, J. and Filipsson, O. 1985. Ecological testfishing with the Lundgren gillnets of multiple mesh size: the Drottningholm technique modified for Newfoundland Arctic char populations. Rep. Inst. Fresw. Res. Drottnigholm 62: 12-35.
Jensen, J. and Hesthagen, T. (1996). Direct estimates of the selectivity of multimesh and series of single gillnets for brown trout, Salmo trutta. J. Fish Biol. (in press).
Kurkilahti, M. and Rask, M. 1995. A comparison of perch and roach catches from gillnet series and multimesh gillnets to population data obtained from marking and recapturing. Fisheries Research
Lee, R.M. 1920. A review of the methods of age and growth determination in fishes by means of scales. Fishery Invest. Lond. ser. II 4 (2), 1-32.
Rosseland, B.O., Balstad, P., Mohn, E., Muniz, I.P., Sevaldrud, I. and Svalastog, D.. 1979. Bestandsundersrkelser DATAFISK-SNSF-77. Presentasjon av utvalgskriterier, innsamlingsmetodikk og anvendelse av programmet ved SNSF-prosjektets prrvefiske i perioden 1976-79. (Fish population studies, DATAFISH-SNSF-77. Presentation of criteria for lake selection, data collection and use of data program in the SNSF-projects test fishing program in the period 1976-79. SNSF-project, Technical Report TN 45/79, 63 pp. + appendix. (In Norwegian).
SFT 1983. Overvlking av langtransportert forurenset luft og nedbrr. Lrsrapport 1982.(Monitoring of long range transported pollutants in air and precipitation. Annual report 1982). Statlig program for forurensningsovervlking. Rapp. 108/83, SFT, Oslo.
SFT 1993. Overvlking av langtransportert forurenset luft og nedbrr. Lrsrapport 1992. (Monitoring of long range transported pollutants in air and precipitation. Annual report 1992). Statlig program for forurensningsovervlking. Rapp. 533/93, TA 981/1993, SFT, Oslo.
Wathne, B.M., Patrick, S.T., Monteith, D and Barth, H. (eds.) 1995. AL:PE 1 Report for the period April 1991 - April 1993. Ecosystem Research Report 9, European Commision Report EUR 16129 EN, 296 p. ISBN 2-87267-129-1
Protocol for fish physiology
Jean-Charles Massabuau, CNRS, Arcachon, France
1. Blood ionic composition:
To avoid intercalibrations, all blood ion analyses will be performed at CNRS, Arcachon, France. CNRS will send a complete package to all MOLAR Fish Groups containing sampling and storing equipment.
1.1 Sampling equipment
In one package for one lake, you have:
- 10 syringes, 2 ml
- 10 yellow needles
- 10 pink needles
- 10 plastic pipettes
- 6 ml of heparin
- 20 tubes for centrifugation (1.5-2.0 ml)
- 10 tubes for blood transfer (2.2 ml). Each tube is numbered.
- No hematocrit tubes. The idea is that if you have a centrifuge for doing hematocrit, you have the corresponding tubes.
- plastic gloves
- 16 microcollection kits for blood and plasma hemoglobin contents.
Each kit contains either 1.25 or 2.5 ml of Drabkin solution. It is toxic as it contains cyanide. So take care although the risk is really minimum with these kits. You can use the provided gloves. See procedure on the video and on the back side of the general cooking list.
1.2 Blood Analysis Protocol (6 very fresh fish)
-Take a very freshly catched fish, check and note its number
- Sample its blood gently!
- Separe some blood for an hematocrit (if you can do it)
- Separe 10 L of blood for the hemoglobin measurement by using the provided kit. Do not freeze. The kit is already numbered. Note it
- Centrifuge about 1.5-2 ml of blood
- Collect the plasma with a plastic pipette
- Separe 10 L of plasma for the hemoglobin measurement by using the provided kit. Do not freeze. The kit is already numbered. Note it.
- Put the remaining plasma in the numbered 2.2 ml tubes and note the corresponding numbers
- Keep the plasma tubes in the cold. Freeze as soon as possible.
Contact us before sending
Jean-Charles MASSABUAU Tel: (33) 56 22 39 25/ (33) 56 22 39 20
Laboratoire de Neurobiologie et Physiologie Comparees Fax: (33) 56 83 03 50
Universite Bordeaux I et CNRS e-mail: massabuau@lnpc.u-bordeaux.fr
Place du Dr Peyneau
33120 Arcachon France
Jean FORGUE Tel: (33) 56 83 03 28
Laboratoire de Neurobiologie et Physiologie Comparees Fax: (33) 56 83 03 50
Universite Bordeaux I et CNRS e-mail: forgue@lnpc.u-bordeaux.fr
Place du Dr Peyneau, 33120 Arcachon France
1.3 MOLAR Scanning and Electronic Microscopy of fish gills (cooking list for 5 fish)
In one package for one lake, you must have:
- tweezers and scissors You must have your own material
- razor blades
- 3 x 10 ml syringes
- 2 empty bottles numbered 2 and 3
- 1 field dissection table
- 6 small transfer tubes (numbered)
- a kit to prepare extratemporaneously 100 ml of cacodylate buffer
- 1 ampula (10 ml) of glutaraldehyde
- 1 ampula (2 ml) of osmium tetroxide (Take care !!!!!, toxic, to use with latex gloves)
- latex gloves
- plastic pipettes
- 1 syringe with 6 ml of distilled water
- 50 ml of ethanol 70 %
On the field, prepare solutions 1 and 2 below, USE LATEX GLOVES:
Cacodylate buffer: Solution 1 in bottle 1,
- Reciepe. To prepare a few hours before the test fishing.
You have a plastic bottle with 97.5 ml of distilled water in it. There are 1.07g of sodium cacodylate in the small plastic can that is scotched on it. Empty it in the distilled water and put also the can (and its plug) in the water. Sheake it to dissolve the powder. Add the 2.5 ml of HCl 0.2 N provided in the 2 ml syringe. Sheake it. The volume of acid has been calculated to adjust the pH of the solution at 7.4.
Solution 2 in bottle 2: break one 10 ml ampula of glutaraldehyde and mix with 10 ml of cacodylate buffer by using 10 ml syringes,
Solution 3 in bottle 3: break one 2 ml ampula of osmium tetroxide and mix with 6 ml of distilled water by using 10 ml syringes.
1.4. Sampling protocol; (5 very fresh fish)
-Take a very freshly catched fish, check and note its number
-Dissect the first gill arch, put it on the small "field dissection table" provided.
-Section it in portions that contain from 2 to 4 pairs of filaments, the arch tissue must be cut with the provided razor blades leaving the pairs of filaments attached by the septum.
-Take at least 6-8 samples per animal.
-Put them in 1 tube, add 2 ml of solution 2 and leave 2 hours.
-Two hours later, empty the tube with a plastic pipette, filled with solution 1 once, empty the tube, filled with solution 1 again.
Leave it like that for 12-24 hrs at 10 C in theory (in fact in a fresh place and at least in the shadow). No more than 24 hrs!!!!!
-12-24 hrs empty the tubes with the plastic pipettes and add 1.5 ml of solution 3.
Leave it like that for 1 hr
-1 hr later, empty the tube, rinsed 10 times in ethanol 70 % (empty the tube, fill the tube gently, shake the tube gently, empty....)
-Finally, store the samples in ethanol 70 %. Seal the tubes and send them. Do not freeze. Contact us before sending
Jean-Charles MASSABUAU
Tel: (33) 56 22 39 25 / (33) 56 22 39 20; Fax: (33) 56 83 03 50e-mail: massabuau@lnpc.u-bordeaux.fr
Suzanne Dunel / Claudine Chevallier Tel: (33) 88 10 69 00; Fax: (33) 88 10 69 06
Centre dEcologie et Physiologie Energetiqu, 23 rue Becquerel, 67097 Strasbourg, FRANCE
Protocol for fish histology
Rudolf Hofer, Institute of Zoology, UIBK, Austria
1. Sampling the fish
- Fish are caught by gill nets. If possible fish should not be exposed for more than 3-4 hours in gill nets and only live fish can be used for histological samples.
- Select 20 males, preferably in the age of 5-8 years, and keep them until dissection under aerated conditions and at temperatures similar to those of the lake. Females should be only used if not enough males are available (during autumn the liver of females is more affected by vitellogenesis than by environmental parameters). Minimum number of fish: 12 males.
2. Dissection of the fish
Dissect the fish immediately at the lake site.
You will get a complete set containing:
a. Tubes with formalin for tissues (all tissue samples of one fish are collected in one tube)
b. Empty tubes for otoliths
c. Figures for fish dissection
d. Protocol form
* Kill the fish by a blow on the head (not too hard) and record its weight and total length. If no balance is available in the field and a transport of live fish to the laboratory under optimum conditions is impossible, take only the length of the fish.
The dissection should be performed within 10 min after you have killed the fish! In consequence, kill the first fish and dissect it immediately, then kill the second fish and collect the tissues, and so on.
* Cut the second gill arch of one side by scissors and transfer it to formalin. Dont squeeze the gill filaments!
* Open the body of the fish, dissect a portion of the liver and transfer it to formalin (about 0.5 cm3, not more than 1 cm3). Dont squeeze the tissue!
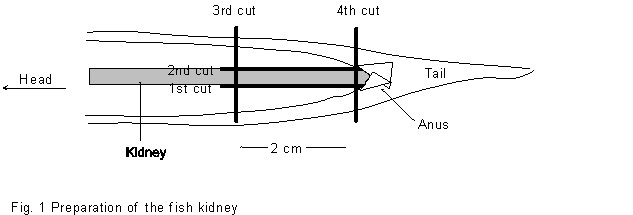
* Cut carefully the caudal portion of the kidney by a scalpel (see Figure 1) without squeezing the tissue.
* After you have collected the tissue of all fish, dissect the otolith bones of the fish and store them in a tube. If you are not familiar with otolith dissection cut the head of the fish, sign it with its number, freeze it and send it to Innsbruck.

3. Storage and transport of samples
All samples should be stored at room temperature and can be sent to Innsbruck by normal
post.
IMPORTANT
Use only live fish
Tissues sampling within 10 min after you have killed the fish
Dont squeeze the tissues
Do not freeze the tissue samples !!!
4. Protocol form
Lake:
Date of sampling:
Maximum time at which fish were exposed in gill nets:
Maximum time between capture and dissection:
Conditions during storage of live fish:
Problems:
Responsible person:
|
No. of fish |
Sex |
Total body length (mm) |
Total body weight (g) |
Remarks |
|
1 |
Male |
231 |
66.3 |
Skin lesions |
|
2 |
Male |
192 |
48.0 |
Parasites .......... |
The samples should be returned to: Rudolf Hofer, Innsbruck
|
Rudolf Hofer University og Innsbruck Institute of Zoology Technikerstrasse 25, A-6020 Innsbruck, Austria |
Phone: 43 512 2186183 Fax: 43 512 2182930 Rudolf.Hofer@uibk.ac.at |
Protocol for heavy metals in fish
Sigurd Rognerud, NIVA, Norway
1. Sampling
To get an estimate of adjusted means with an acceptable statistical significance it is necessary to collect 25 fish of different length in the intreval 15-30 cm from each lake. Record total length, weight and sex. Collect scales or otoliths for age estimates. Remove approximately 40g of bone and skinnless dorsal axial muscle, liver and kidneys from each fish, put it separatly in polyethylene bags and store it in the freezer.
2. Shipment
All samples must be carefully packed and shipped to NIVA in a frozen condition. Inform the reciever so the package could be picked up before it thaws.
3. Metal analyses
Approximately 1g muscle is digested in a 4: 1 nitric-perchloric acid mixture under pressure. After dissolution, samples are analyzed for mercury by a atomic absorption spectrophotometer equipped with a hydrid generator (detailed description given in Fjeld & Rognerud 1993). Lead and cadmium are analysed by atomic absorption spectrometry using a graphite furnace.
The frozen samples should be returned to NIVA after direct contact and agreement by either phone call, Fax or E-mail:
Sigurd Rognerud1) or Leif Lien2)
NIVA
P.O. Box 173 Kjelsls
N-0411 Oslo, Norway
Phone: 1) 47-62576400 2) 47-22185100
Fax: 1) 47-62576653 2) 47-22185200
sigurd.rognerud@niva.no
leif.lien@niva.no
4. Reference
Fjeld, E. and Rognerud, S. (1993). Use of path analyses to investigate mercury accumulation in brown trout (Salmo trutta) in Norway and the influence of environmental factors. Can. J. Fish. Aquat. Sci. 50: 1158 - 1167.
Protocol for organic micropollutants in fish
Joan O. Grimalt, Lourdes Berdie, Barcelona, Catalonia, Spain
1. ORGANOCHLORINATED COMPOUNDS IN FISH TISSUES (MUSCLE AND LIVER)
1.1 Compounds to be determined.
* Hexachlorobenzene
* Hexachlorocyclohexanes (namely a and g isomers)
* DDTs (namely, pp'-DDE and pp'-DDT)
* Polychlorobiphenyls (congeners Nos. 28+31, 52, 101+84, 118+149, 153, 138+163+160 and 180)
* Polycyclic aromatic hydrocarbons (PAH; 63 individual compounds)
1.2 Other measurements.
* Water and lipid content
1.3 Materials and reagents.
-Self adhesive labels
-Pen
-Big box to keep the samples when dissected
-Field fridge
-Glass bottles
-Recipient for cleaning and rinsing residues
-Latex gloves (only for cleaning the material in the soap, for safety reasons). Not to dissect the fishes.
-Plastic tray.
-Scalpel
-Tweezers
-Scissors
-Aluminum foil
-Sulphuric acid for analysis grade (Merck)
-Potassium hydroxide (for analysis, Merck)
-Iso-octane (for trace organic analysis, Merck)
-Acetone (for trace organic analysis, Merck)
-Distilled water
-Lake water
-Soap (Extran MA01, ref. 7555.1000, alkaline, Merck)
-Internal standards: Tetrabromobenzene and PCB 209
1.4 Cleaning.
Correct cleaning of the material is a very important step in the analysis of organic compounds. Plasticizers are present in most recipients and material of common use (e.g., gloves, bottles -distilled water is usually kept in plastic bottles-, ...). The hands and hair can also be a source of organic compounds in the sample. For this reason the cleaning procedure needs to be followed carefully during sampling and analysis.
1. Find a flat horizontal surface to work comfortably.
2. Put the gloves on.
3. Fill the plastic tray with EXTRAN solution (20 g per litre of distilled water).
4. Clean the glass bottle with this solution.
5. Rinse the glass bottle with lake water (3 times)
6. Fill the glass bottle with lake water.
7. Spread an aluminium sheet over the grass.
8. Put in the tray the material to be used and clean it.
9. Once cleaned, rinse it with the lake water stored in (6).
IMPORTANT: Do not throw the first rinsing water nor the cleaning mixture in the catchment. Store it in a recipient and throw it when being back in the lab. EXTRAN products can be discarded in the inorganic salt recipient for residues of the lab.
10. Put the material on the aluminium sheet spread in (7)
11. Cover the clean material with aluminium if not to be used immediately.
1.5 Sample amount.
5 g. of muscle. 0.5 g of liver.
1.7 Transport.
The fish tissues, protocol form and the list of analyses performed to each fish should be placed in a box containing dry ice and sent to Barcelona. Before mailing contact directly with Joan Grimalt, Pilar Fernandez or Rosa Vilanova:
Department of Environmental Chemistry (CID-CSIC)
Jordi Girona, 18
08034-Barcelona
Catalonia. Spain
Phone: 34 3 400 61 22 or 34 3 400 61 00
Fax 34 3 204 59 04
e-mail: jgoqam@cid.csic.es
lbrqam@cid.csic.es
mrvqam@cid.csic.es
pfrqam@cid.csic.es
1.8
Analyses (extraction).The tissues are freeze-dried in an oil-free freeze-drier and Soxhlet extracted with (4:1) n-hexane-dichloromethane for 18 hours. An aliquot (10%) of this extract is used to measure total extractable lipid weight after evaporation to dryness. Water content is determined by weight difference prior and after freeze-drying.
1.9 Analyses (organochlorinated compounds in muscle and liver).
The extract is spiked with TBB and PCB209 in order to assess the analytical recovery. Then it is reduced to 2 ml of n-hexane and cleaned up with agitation with sulphuric acid. After vigourous stirring in a Vortex (2 min) the two layers are decanted by centrifugation for removal of the sulphuric acid. The n-hexane extract is neutralized by washing (three times) with Milli-Q water for pH neutralization. The n-hexane is then concentrated under vacuum to a small volume, e.g. 50 l, for instrumental analysis.
1.10 Analyses (polycyclic aromatic compounds).
After lyophilisation, the sample is saponified with 6 N NaOH and then extracted with (4:1) n-hexane-dichloromethane to obtain the total organic extract. The extract is vacuum evaporated to 0.5 ml and fractionated by column chromatography using columns filled with 5% water deactivated silica (top) and alumina (bottom). The first fraction (n-hexane) is discarded. The second fraction (n-hexane:dichloromethane) is collected and evaporated (vacuum rotary evaporation and nitrogen stream) to dryness for PAH analysis.
1.11 Instrumental analysis of organochlorinated compounds.
A gas chromatograph Hewlett-Packard Model 5890 (Palo Alto, CA, USA) equipped with a 63Ni electron capture detector and a split/splitless injector is used. This equipment is provided with a DB-5 column (5% phenyl-95% methylpolysiloxane, 25 m length, 0.25 mm i.d., 0.25 mm film thickness; J&W Scientific, Folsom, CA, USA). Helium is the carrier gas (30 cm/s). The samples (2 l) are introduced with an automatic injector Hewlett-Packard Model 7673A in the splitless mode. Injection and detector temperatures are 270 and 310sC, respectively. Oven temperature is programmed from 60 to 300sC at 6sC/min with a final holding time of 10 min. The make up gas is nitrogen (60 cm/s).
1.12 Instrumental analysis of polycyclic aromatic hydrocarbons.
A Carlo Erba GC8000 Series coupled to a mass spectrometer Fisons MD800 is used. This instrument is equiped with a 30 m HP-5 column coated with 5% phenyl methyl silicone. The oven temperature program is from 90 to 310sC at 4sC/min, held for 10 min. Injection and transfer line temperatures are 280 and 300sC, respectively. Helium is the carrier gas (50 cm/s). Data are acquired in the electron impact mode with an electron energy of 70 eV and the operation is in selected ion monitoring. The injection is in the splitless mode (1 l injected; hot needle technique), the split valve being closed for 48 s.
1.13 Quantitation.
Authentic standards of hexachlorobenzene, a-, g- and d-hexachlorocyclohexane, op'-DDE, pp'DDE, op'-DDD, pp'-DDD, op'-DDT, pp'-DDT and the polychlorobiphenyl congeners Nos. 28, 52, 101, 118, 138, 153 and 180 are used. Calibration curves (detector response vs amount injected) are performed for each compound. The polycyclic aromatic hydrocarbons are analyzed by reference to standards of fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[k]fluoranthene, pyrene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, benzo[ghi]perylene and dibenzo[ah]anthracene. The range of linearity of the detector is evaluated from the curves generated by representation of detector signal/amount injected vs amount injected. All measurements are performed in the ranges of linearity found for each compound. In some cases, re-dilution and re-injection are performed to fit within the linear requirements.
1.14 Compound identification.
Structural identification is confirmed by analysis of selected samples by gas chromatography coupled to mass spectrometry in the chemical ionization mode and record of the negative ions. The chromatographic conditions are the same as described above. A DB-5 column was used. Transfer line and ion source temperatures are 300 and 120sC, respectively. The reagent gas is methane. Data are acquired by scanning from 50 to 500 mass units at 1 s per decade. The samples selected for GC-MS analysis must allow to elucidate the composition of all the major peaks present in the gas chromatograms obtained with the ECD.
Protocol for sampling procedure of fish blood and tissue
The following working protocol is based on the agreed sampling procedure made by the MOLAR Fish Group. A videotape showing all fish sampling procedure for blood and tissue was made by CNRS during the Fish Group Workshop in Arcachon, France, June 1996.
Two peoples should attend. Before starting, prepare aluminium foil, paper and dissection tools, and the content of the packages sent by the different laboratories.
1- Blood sampling (6 fish). Heparanized a 2 or 5 ml syringe. Take the blood sample from the ventral aspect of the tail. You should get 1.5-2 ml of blood.
- Put off the needle and empty an hematocrit tube, seal it with the hematocrit paste, centrifuge.
- Collect 10 L of blood for the hemoglobin measurement by using the provided kit. Do not forget to wipe the excess blood from outside of capillary pipette. Note the number written on the kit. Wrap it in an aluminium foil.
- Place the remainder of the blood in a 1.5-2 ml centrifuge tube and separe the plasma from the pellet (red blood cells and leucocytes; 9-10 min and 10 000 rpm).
- Collect 10 L of plasma for the hemoglobin measurement by using the provided kit. Note the number written on the kit. Wrap it in an aluminium foil
- Collect plasma in the plasma transfer tubes frozen
2- Weigth the fish and measure its length from the noose to the end of the tail
3- Excise the 1st gill arch for the scanning and electron microscopy (5 fish). Cut 6 to 8 segments
with the razor blade by using the small dissection table. Cut the bones. Place the samples in the corresponding tubes, notice at what time you did it and the corresponding number. Already note the time at which you will have to change the bath. Follow procedure described by CNRS!
4- Excise the 2nd gill arch for histology. Put it in formalin. (20 fish).
5- If the gall bladder is fairly full of bile, empty it with a 1ml syringe. Fill a centrifuge tube, tag it
(20 fish) frozen
6- Cut the liver: One little piece in formalin (20 fish). Cut the remaining in two pieces. One must be used for the heavy metals (25 fish) and placed in a plastic bag frozen. The other one is for the organics (20 fish) and it must be kept in aluminium. Tag the aluminium staff with a sticker. frozen
7- Kidney: In the posterior part of the open abdomen, cut 1 cm of kidney and put it in formalin with the liver (20 fish). In the anterior part, cut 5 cm of kidney for the heavy metal (25 fish) and put it in a plastic bag frozen
8- Muscles. With a scalpel, cut along the fish length just above the lateral line. Cut verticaly in the anterior and posterior region. Then, starting from the anterior, peel off the skin with a tweezer. Cut in the middle. Separe the flesh from the body by cutting from the back. Put one piece in the plastic bag for the heavy metals (25 fish) frozen. Put the other one in an aluminium foil for the organics (20 fish), add a sticker frozen
9- Scales. Turn the animal onto the other side. Eliminate the mucus and then collect some scales
with a scalpel or your knife. Put the scales in the special tiny envelope. Don't forget to fill the corresponding questionnary.
10- Otoliths. Take your best knife and open the roof or the head in order to see the brain. Sample the otoliths. You need two big ones. Put them in a plastic bags that you place into the envelope. If you can not find them, keep the head and send it like that to NIVA.
11- Congratulation, you have finish this fish. There is no more noise from the centrifuge but did you collect the plasma? Measure the hematocrit? Check the bath for the scanning and electron microscopy? You noted the fish number. Nothing to do ??? Start with a new fish....
Samplig protocol forms for lakes Gossenkollesse, Redo and Rvre Neadalsvatn.
|
Fish Code |
Fish weight |
Fish length |
Sex |
Age |
Remarks |
|
(1) |
|||||
|
(2) |
|||||
|
(3) |
|||||
|
(4) |
|||||
|
(5) |
|||||
|
(6) |
|||||
|
(7) |
|||||
|
(7) |
|||||
|
(8) |
|||||
|
(9) |
|||||
|
(10) |
|||||
|
(11) |
|||||
|
(12) |
|||||
|
(13) |
|||||
|
(14) |
|||||
|
(15) |
|||||
|
(16) |
|||||
|
(17) |
|||||
|
(18) |
|||||
|
(19) |
|||||
|
(20) |
Samplig protocol forms for the lakes where only 5 fish specimens will be taken.
|
Fish Code |
Fish weight |
Fish length |
Sex |
Age |
Remarks |
|
(1) |
|||||
|
(2) |
|||||
|
(3) |
|||||
|
(4) |
|||||
|
(5) |
List of analyses available for each fish
|
Fish Code |
Fish Population |
Blood ionic composition |
Scanning electron microscopy of gill filaments |
Electronic microscopy of gill filaments |
Fish histology |
Heavy metals |
Organics |
Remarks |
|
(1) |
||||||||
|
(2) |
||||||||
|
(3) |
||||||||
|
(4) |
||||||||
|
(5) |
||||||||
|
(6) |
||||||||
|
(7) |
||||||||
|
(8) |
||||||||
|
(9) |
||||||||
|
(10) |
||||||||
|
(11) |
||||||||
|
(12) |
||||||||
|
(13) |
||||||||
|
(14) |
List of analyses available for each fish
|
Fish Code |
Fish Population |
Blood ionic composition |
Scanning electron microscopy of gill filaments |
Electronic microscopy of gill filaments |
Fish histology |
Heavy metals |
Organics |
Remarks |
|
(15) |
||||||||
|
(16) |
||||||||
|
(17) |
||||||||
|
(18) |
||||||||
|
(19) |
||||||||
|
(20) |
||||||||
|
(21) |
||||||||
|
(22) |
||||||||
|
(23) |
||||||||
|
(24) |
||||||||
|
(25) |